Regulatory Submissions.
Simplified.
14 regulatory services. AI-powered workflows. Expert guidance.
Streamline your medical device regulatory submissions with AI-powered workflows. From FDA 510(k) to De Novo, PMA to global markets—intelligent automation, automated compliance checks, and expert guidance at every step.
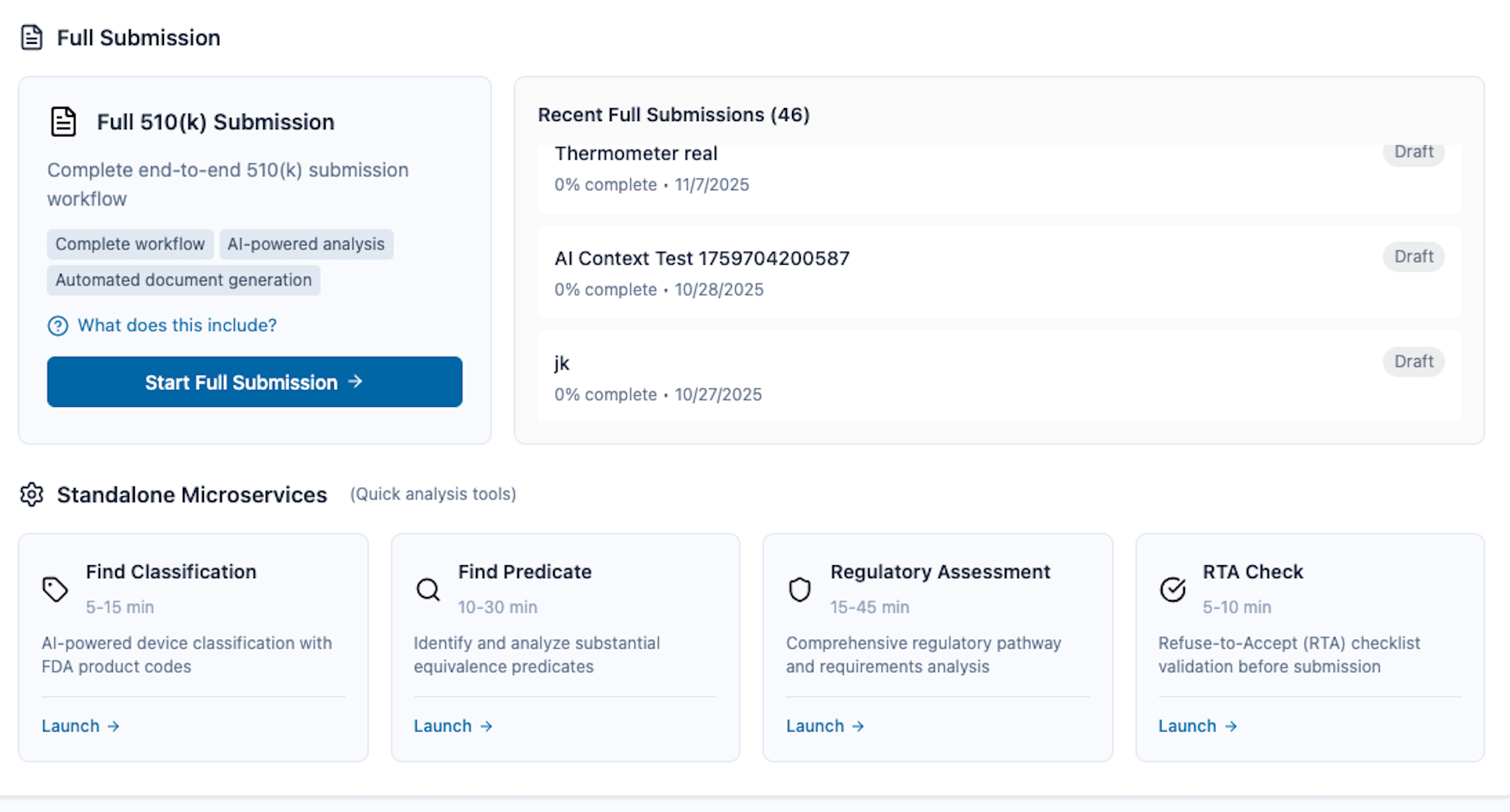
Complete Regulatory Platform
14 services covering FDA and global markets. One intelligent platform for all your regulatory needs.
FDA Regulatory Services
FDA 510(k)
Traditional 510(k) submission for medical devices with 90-day FDA review. Complete workflow from device classification to final submission package.
De Novo Classification Request (eSTAR)
For novel, low/moderate risk devices. eSTAR use mandatory Oct 1, 2025
Q-Submission (Pre-Sub)
Structured questions + meeting request + briefing book for sponsors
513(g) Request
Formal device classification determination - short dossier + cover letter
PMA Supplements
180-day, Real-Time supplements with repeatable templates/checklists
Humanitarian Device Exemption (HDE)
Targeted niche submissions with PMA scaffolding overlap
IDE Submissions
Clinical study protocols with risk assessment and monitoring templates
Breakthrough Device Designation
Designation request dossier for breakthrough technologies
Safer Technologies Program (STeP)
Safety-focused designation similar to Breakthrough program
CLIA Waiver (IVDs)
Dual 510(k)+CLIA or standalone waiver with study plans
Global Regulatory Services
EU MDR Technical Documentation
Annex II + III PMS/PSUR scaffolding for Notified Body-ready tech files
UKCA Dossiers
UK transition & future regime with EU MDR content reuse + UK specifics
Health Canada MDL
Class II–IV straight-through forms + attachments for quick wins
Australia TGA ARTG
Classification + evidence mapping with audit support
Built for regulatory professionals
AI-powered workflows, automated compliance checks, and expert guidance at every step of your submission journey.
AI-Powered Classification
Automated device classification with comprehensive analytics. Identify product codes, regulation numbers, and classification pathways instantly.
Intelligent Predicate Finding
AI-driven predicate device identification and substantial equivalence analysis. Compare your device against thousands of cleared devices.
Automated RTA Checks
Pre-submission readiness checks to prevent Refuse to Accept (RTA) letters. Automated compliance validation before submission.
Regulatory Assessment
Comprehensive regulatory requirements analysis across all 18 eSTAR sections. AI-powered sectionator with intelligent content generation.
eSTAR Editor
Professional FDA eSTAR submission editor with intelligent drafting, review, and export capabilities. Generate submission-ready packages.
Evidence Planning
Intelligent evidence requirement mapping. Upload, organize, and manage all supporting documents with automated compliance tracking.
Compliance Advisor
AI-powered compliance review and recommendations. Get expert guidance on regulatory requirements and best practices.
Final Review & QA
Comprehensive submission readiness assessment with detailed QA reports. Ensure your submission meets all FDA requirements.
Export & Package
Generate complete submission packages with all required documents, properly formatted and ready for FDA submission.
How Cruxi Ensures Highest Quality
Every submission is built on a foundation of accuracy, compliance, and expert validation. Here's how we guarantee the highest quality standards.
FDA-Guidance Aligned Intelligence
Our AI models are trained on current FDA guidance documents, regulations, and successful submission patterns. Every recommendation is backed by regulatory precedent and official FDA guidance, ensuring your submission aligns with FDA expectations.
Multi-Layer Validation System
Every section undergoes automated compliance checks, cross-referencing against FDA requirements, predicate device data, and regulatory databases. Our validation system catches inconsistencies before they reach FDA reviewers.
Zero Hallucinations Guarantee
Every claim, citation, and data point is traceable to its source. Our AI never invents information—it synthesizes from verified regulatory databases, FDA guidance, and your uploaded documents with full citation tracking.
Automated RTA Prevention
Pre-submission readiness checks validate completeness, format compliance, and required documentation. Our system identifies potential RTA issues before submission, saving months of delays and resubmission cycles.
FDA Reviewer Psychology Insights
Built-in understanding of how FDA reviewers evaluate submissions. Our platform structures content in ways that facilitate reviewer comprehension, highlighting key information and reducing review friction.
Real-Time Regulatory Updates
Our platform continuously monitors FDA guidance updates, regulation changes, and industry best practices. Your submissions automatically incorporate the latest regulatory requirements and standards.
Expert Review Integration
Optional expert regulatory affairs review at critical checkpoints. Our network of FDA-experienced regulatory professionals provides human validation of AI-generated content, ensuring both technical accuracy and strategic positioning.
Continuous Quality Improvement
Every submission outcome feeds back into our quality system. We analyze FDA feedback, acceptance rates, and review patterns to continuously refine our AI models and validation rules, ensuring each submission is better than the last.
Enterprise-Grade Security & Compliance
SOC 2 Type II audited infrastructure with HIPAA and GDPR compliance. Your sensitive regulatory data is protected with bank-level encryption, access controls, and audit trails. Quality also means security.
Start your regulatory submission today
Join medical device companies using AI-powered workflows to streamline FDA and global regulatory submissions. One platform, 14 services, infinite possibilities.
4-5 hours. Complete workflow. Expert guidance.