Regulatory Assessment
Comprehensive AI-powered regulatory assessment with 17-section analysis. Sectionator analyzes your device across all eSTAR sections, identifying applicable standards, guidances, special controls, testing requirements, and compliance needs.
17-section analysis. AI-powered insights. Complete compliance.
17-Section Comprehensive Analysis
Sectionator analyzes your device across all eSTAR sections, providing advisory guidance on what applies to your device based on classification, regulation data, and device characteristics.
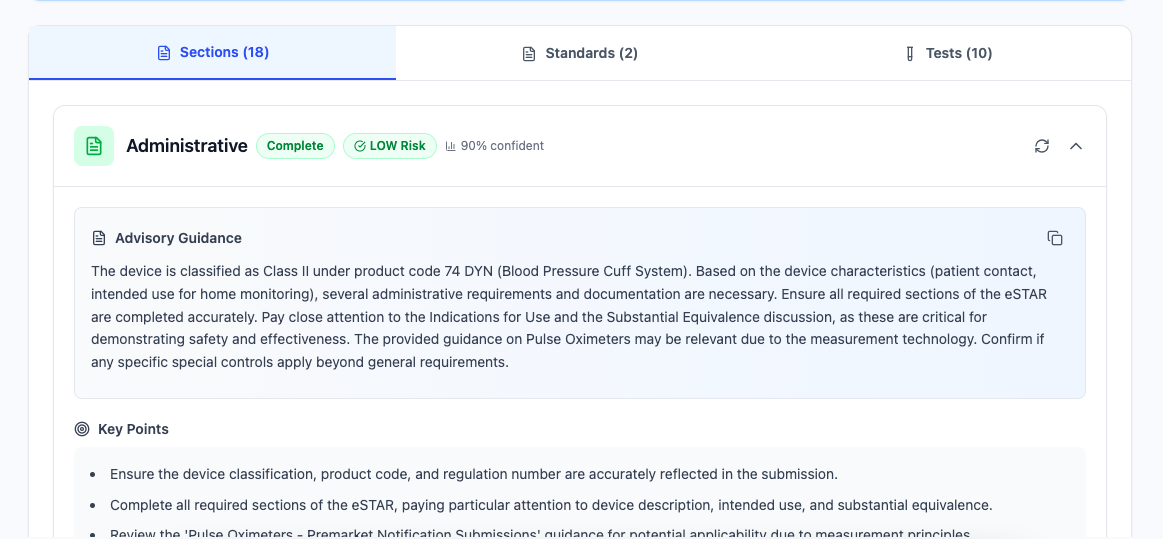
1. Summary at a Glance
Executive overview of device classification, regulatory pathway, and key requirements.
2. Regulation Fit Rationale
Detailed analysis of how your device fits within applicable FDA regulations.
3. Class, Exemptions & Conditions
Device class determination, exemption status, and any special conditions.
4. Special Controls
Identification and mapping of all applicable special controls for your device.
5. Guidance Documents
Comprehensive list of relevant FDA guidance documents with citations and anchors.
6. Standards Map
FDA-recognized standards applicable to your device with recognition status and editions.
7. Testing Matrix
Required test methods, configurations, acceptance criteria, and status tracking.
8. Software & Cybersecurity
Software documentation requirements, level of concern, IEC 62304 compliance, and cybersecurity needs.
9. Sterilization
Sterilization validation requirements, SAL documentation, and package integrity testing.
10. Biocompatibility
ISO 10993 series requirements based on patient contact type and duration.
11. Labeling & UDI
Labeling requirements, IFU needs, UDI compliance, and packaging labels.
12. Human Factors
Usability testing requirements, especially for home use or OTC devices.
13. Clinical Evidence
Clinical data requirements, protocols, IRB, informed consent, and data extracts.
14. Market Landscape
Analysis of competitive landscape and market positioning for your device.
15. International Alignment
Harmonization with international regulations (EU MDR, Health Canada, etc.).
16. Risks & Gaps
Identification of regulatory risks, data gaps, and areas requiring attention.
17. Conflicts & Considerations
Contradictions, missing information, and important considerations for FDA review.
Advanced Capabilities
Comprehensive AI-powered features that ensure complete regulatory compliance.
smart_toyAI-Powered Analysis
Senior FDA regulatory advisor AI analyzes each section with comprehensive device information, classification data, and regulation cards to provide expert-level guidance.
ruleStandards & Guidances
Identifies all applicable FDA-recognized standards with exact editions and relevant guidance documents with section anchors, citing why each applies to your device.
scienceTesting Requirements
Comprehensive testing matrix identifying required test methods, configurations, acceptance criteria, and status tracking based on evidence bundles.
checklistEvidence Tracking
Cross-references evidence bundles to determine what's already done versus what's missing, providing clear status for each requirement.
descriptioneSTAR Section Mapping
References eSTAR catalog CH IDs (e.g., "CH4.02", "CH5.01") and identifies what content needs to be drafted in each section and why it's required.
warningRisk Identification
Identifies risks, gaps, conflicts, and areas FDA will scrutinize, helping you address potential issues before submission.
device_hubDevice Characteristics
Analyzes device characteristics (software, wireless, patient contact, sterile, etc.) to determine conditional requirements automatically.
verifiedCitation Management
Every claim includes citations referencing regulations, guidances, and standards with anchors when possible, ensuring traceability.
Why Choose Cruxi Regulatory Assessment?
Senior FDA regulatory advisor AI with comprehensive regulatory knowledge.
psychologyFDA Reviewer Psychology
Understands FDA reviewer expectations and structures content to facilitate review, reducing the likelihood of additional information requests.
updateFreshness Layer
Automatically retrieves latest guidances and standards, ensuring you're always working with current FDA-recognized editions and active guidance documents.
speedParallel Processing
Run all 17 sections in parallel for faster assessment, or sequentially with rate limiting for controlled processing.
auto_awesomeAdvisory Guidance
Provides actionable advisory guidance on what applies to your device, what you need to do, and what requirements exist—not just draft text.
Ready for Comprehensive Regulatory Assessment?
Get complete 17-section analysis with AI-powered insights and expert-level regulatory guidance.
Start Regulatory Assessment Now