FDA eSTAR Editor
Professional FDA eSTAR submission editor with intelligent AI drafting, real-time analysis, quality scoring, and compliance checking. Edit sections with AI assistance, track changes, and generate submission-ready packages formatted for FDA eSTAR submission.
Intelligent drafting. Real-time analysis. Submission-ready.
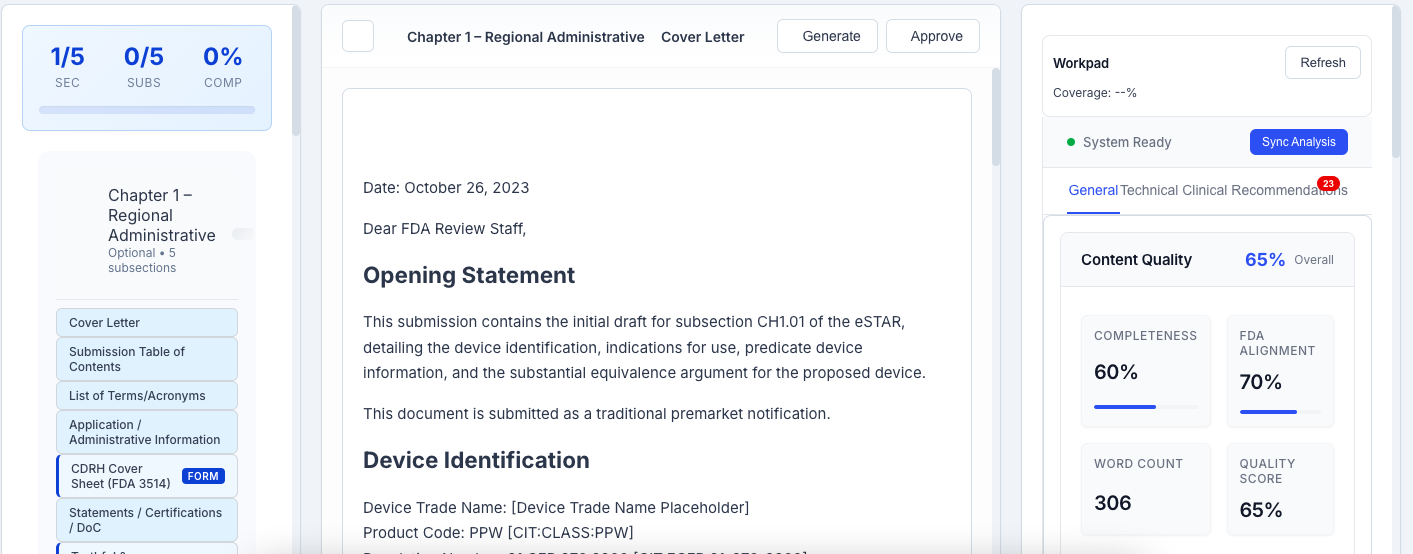
Comprehensive Editor Sections
Professional eSTAR editor with intelligent drafting capabilities across all required submission sections.
Administrative Forms Package
FDA Forms 3514, 3881, Truthful & Accuracy Statement, Cover Letter with required signatures.
Substantial Equivalence Core
Device Description, Intended Use, SE Comparison Table & Rationale with comprehensive analysis.
Software Documentation
SRS/SDS, Architecture, Risk Analysis, Level of Concern, IEC 62304 Compliance documentation.
Cybersecurity Plan
Threat Model, SBOM/SOUP List, Vulnerability Management, Update/Patch Strategy.
Non-Clinical Rationale
Justification for absence of clinical data based on bench testing and predicates.
Special Controls Mapping
Matrix linking each special control to provided evidence and standards.
Design Control Summary
Design changes summary for Special 510(k) route with comprehensive documentation.
Submission Cover Package
Executive summary, table of contents, submission overview with professional formatting.
Advanced Capabilities
Enterprise-grade AI services for intelligent drafting and quality assurance.
auto_awesomeIntelligent Co-Pilot
Real-time AI analysis and suggestions as you type. Intelligent content generation with context awareness and regulatory compliance.
editIntelligent Drafting
AI-powered content generation for each section, pre-filled from intake data, assessment results, and evidence bundles.
verifiedQuality Engine
Real-time quality scoring and compliance checking. Identifies issues, suggests improvements, and validates against FDA requirements.
account_treeKnowledge Integration
Knowledge graph integration provides relevant regulatory information, precedents, and best practices as you draft.
historyChange Tracking
Complete version control with change tracking, revision history, and collaborative editing capabilities.
ruleCompliance Validation
Real-time validation against FDA eSTAR requirements, RTA checklist, and regulatory guidance to ensure compliance.
model_trainingAI Model Orchestration
Advanced AI model orchestrator selects optimal models for different tasks—drafting, analysis, quality checking, and compliance validation.
analyticsAnalytics & Metrics
Performance metrics, completion tracking, quality scores, and readiness indicators for each section and overall submission.
Why Choose Cruxi eSTAR Editor?
Enterprise-grade AI services with comprehensive regulatory knowledge.
rocket_launchAI-Powered Intelligence
Million-dollar backend services with intelligent co-pilot, quality engine, and knowledge integration for expert-level drafting assistance.
speedReal-Time Analysis
Instant feedback and suggestions as you type. Real-time quality scoring and compliance checking without interrupting your workflow.
downloadSubmission-Ready Export
Generate submission-ready packages formatted for FDA eSTAR submission with all required documents properly structured.
groupsAdvanced Collaboration
Multi-user editing with role-based permissions, change tracking, and collaborative review workflows.
Ready to Draft Your Submission?
Get intelligent AI drafting assistance with real-time analysis and quality scoring.
Open eSTAR Editor Now