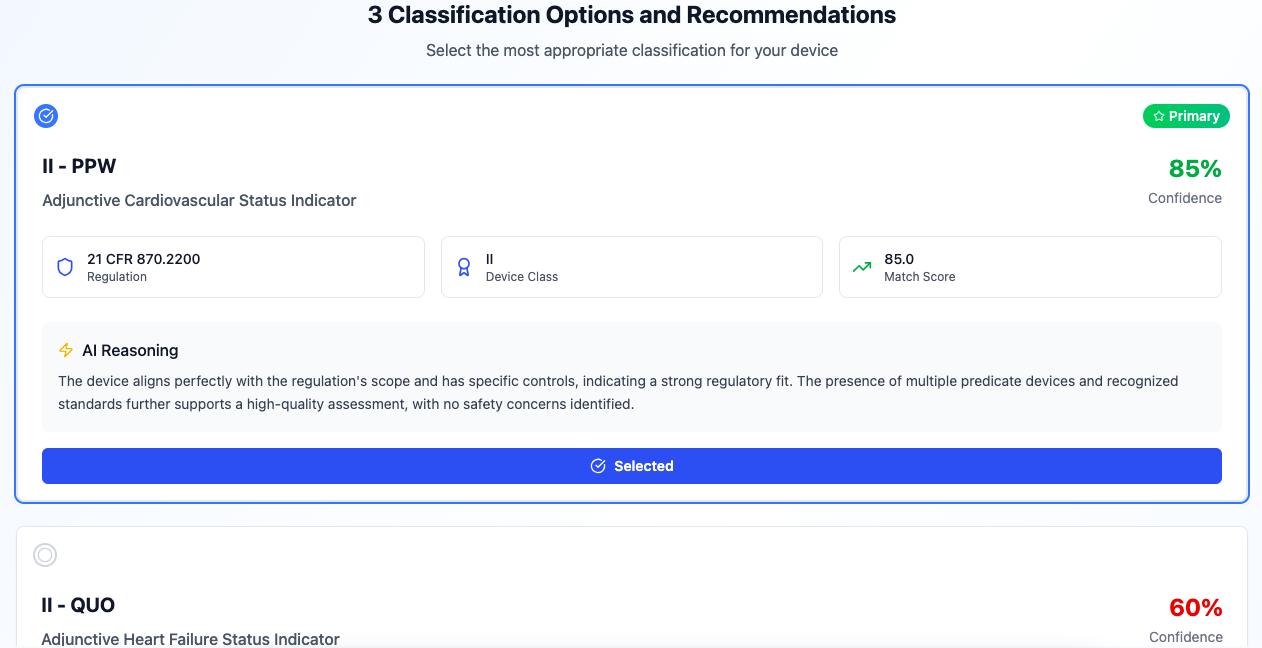
AI-Powered Device Classification
Advanced RegulationFirstAgent v4.0 technology with 7-phase sequential pipeline. Instantly identify product codes, regulation numbers, and classification pathways using tri-channel regulation discovery and comprehensive AI analytics.
100% AI-powered. Comprehensive analytics. Instant results.
7-Phase Sequential Pipeline
RegulationFirstAgent v4.0 uses a sophisticated 7-phase sequential pipeline with 11 AI decision points for comprehensive device classification.
Device Deconstruction
AI analyzes your device description, intended use, technology, and key terms to understand device characteristics and extract structured data.
Search Query Generation
AI generates optimized search queries and predicts CFR Part numbers to efficiently search through regulatory databases.
Regulation Compatibility
AI performs semantic analysis to filter and validate regulations, ensuring compatibility with your device characteristics.
Tri-Channel Discovery
Comprehensive search across Classification Database, 510(k) summaries, and GUDID to find all relevant regulatory information.
Product Code Analysis
AI performs semantic analysis to filter product codes, matching them against device characteristics and intended use.
Multi-Dimensional Scoring
AI-powered 100-point scoring system analyzing evidence quality, standards relevance, and predicate quality for each candidate.
Holistic Assessment
Final AI supervisor review with confidence assessment, risk identification, and next steps generation including De Novo pathway detection.
Advanced Capabilities
Comprehensive AI-powered features that ensure accurate and complete device classification.
auto_awesomeAI Semantic Analysis
Advanced AI semantic analysis for regulation filtering, product code matching, and evidence quality assessment. Understands context and device characteristics beyond keyword matching.
analyticsMulti-Dimensional Scoring
100-point comprehensive scoring system evaluating regulation fit, product code compatibility, standards relevance, predicate quality, and evidence strength for each classification candidate.
searchTri-Channel Discovery
Simultaneous search across Classification Database, 510(k) summaries, and GUDID to ensure comprehensive coverage of all relevant regulatory information and cleared devices.
psychologyEvidence Quality Analysis
AI evaluates the quality and relevance of evidence for each classification candidate, ensuring you have the strongest possible case for your device classification.
routeDe Novo Detection
Intelligent detection of novel devices requiring De Novo classification pathway, automatically identifying when traditional 510(k) route is not applicable.
verifiedComprehensive Audit Trail
Complete audit trail documenting every decision point, search query, and analysis step. Full transparency into how your classification was determined.
speedSmart Caching
Intelligent caching system for performance optimization, reducing classification time while maintaining accuracy and ensuring fresh regulatory data.
filter_listProgrammatic Pre-Filtering
Efficient pre-filtering mechanisms that eliminate incompatible classifications early, reducing processing time and focusing analysis on viable candidates.
Why Choose Cruxi Classification?
Industry-leading AI technology with comprehensive regulatory knowledge.
rocket_launch100% AI-Powered
11 AI decision points across 7 phases ensure comprehensive analysis. No rigid rules—intelligent reasoning adapts to your device characteristics.
databaseComprehensive Database
Access to thousands of cleared devices, product codes, regulations, and standards. Compare your device against the entire FDA database.
precision_manufacturingRegulation-First Architecture
RegulationFirstAgent architecture prioritizes regulatory compliance, ensuring classifications align with FDA expectations and requirements.
insightsConfidence Scoring
Every classification includes confidence scores and risk assessment, helping you understand the strength of your classification decision.
Ready to Classify Your Device?
Get instant, accurate device classification with comprehensive analytics and expert-level insights.
Start Classification Now